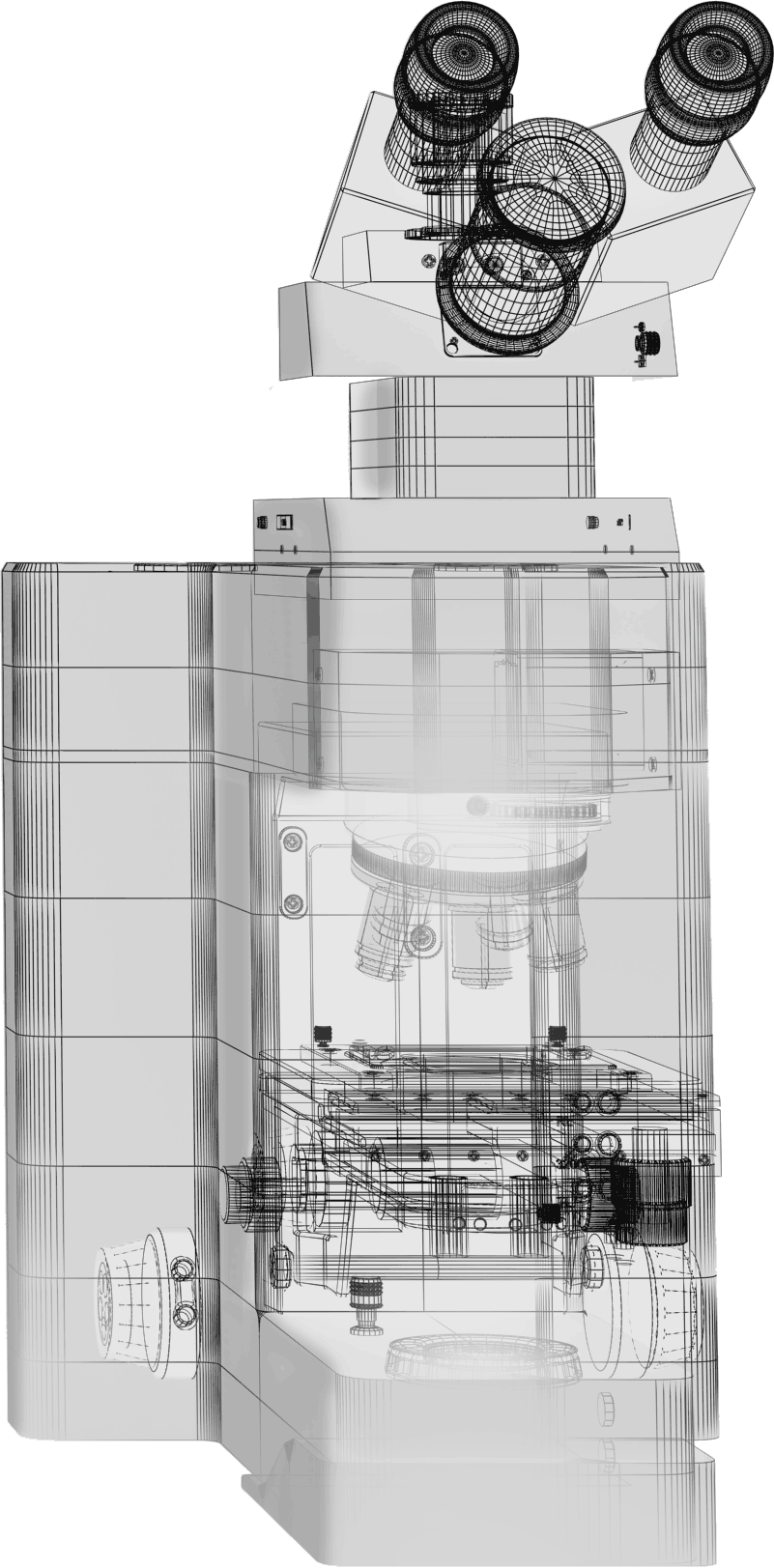
Andor cameras are suitable for a variety of microscopy applications. Choose a slide from the list below to see how our ultra-sensitive detectors can benefit your critical research.
Super Resolution

The ability to take light microscopy beyond the diffraction limit and resolve structures such as vesicles or molecules separated by less than 200 nm is the gift of super-resolution microscopy to cell biology. Among the main super-resolution
techniques are PALM/STORM, STED microscopy, RESOLFT and SIM. In low light conditions image acquisition is a challenging task due to low-signal/high-noise nature of such experiments and the ability of the EMCCD camera to minimize
the camera noise floor, even at fast readout speeds make them ideal. On the other hand with the emergence of brighter and more photo-stable fluorophores, the Zyla 4.2 PLUS, sCMOS cameras make it a compelling choice for super-resolution
microscopy. With their high QE and extremely fast frame rates and field of view, of our new Sona 2.0 and 4.2 are ideal for maximising SNR and therefore localisation accuracy.
Solutions for Super Resolution:
iXon Life 897 | iXon Life 888 | Zyla 4.2 PLUS | Sona 4.2 | Sona 2.0
Gallery:
Further Reading:
Featured Article | Case Study 1 | Case Study 2 | Case Study 3
Flow Measurements
Fluorescence imaging of cells traveling in flow enables image based cell sorting and high-throughput microscopic characterization of extremely large numbers of cells. In addition, non-adherent cells can be imaged in conditions similar
to the blood stream where hydrodynamic forces are known to play an important role in cell morphology and function. The temporal resolution of fluorescence imaging, however, is limited by a finite fluorescence emission rate. For
a cell in fluid flow, this results in a trade-off between exposure time and motion-blur and sets a limit on the achievable signal to noise ratio of the captured image. High speed, high resolution sCMOS cameras are ideally suited,
and the true global shutter functionality of the 5.5 megapixel sensor is essential to avoid readout distortion of the fast moving cells.
Solutions for Flow Measurements:
Neo 5.5 | Zyla 5.5
Gallery:
Further Reading:
Case Study 1
Single Molecule Detection

Recent technical advances in optical detection and manipulation have made the detection of isolated, light emitting probe molecules a reality. Of particular importance to biology is the possibility of direct, real-time visualization
of single biological macromolecules and their assemblies under native physiological conditions, offering great promise for enhancing our understanding of the behaviour, interactions, mechanisms and trafficking of individual biological
macromolecules within the living cell. The raw sensitivity of the iXon range of EMCCD cameras ensures they are capable of detecting single photon events even at fast frame rates making them the ideal detector for the low light
application of single molecule detection.
Solutions for Single Molecule Detection:
iXon Life 897 | iXon Life 888
Gallery:
Further Reading:
Case Study 1 |
Application Note 1
Light Sheet Microscopy

Over the past 10 years, a new fluorescence microscopy technique has come to the fore that is faster and less phototoxic than other microscopy techniques, making it ideal for studying living organisms and the biological processes that
take place within them. Known as Light Sheet Microscopy (LSM) or Selective Plane Illumination Microscopy (SPIM), its key feature is that the illumination is done perpendicularly to the direction of observation. The field of view,
resolution and speed of sCMOS make it an ideal detector for Light Sheet Microscopy.
Solutions for Light Sheet Microscopy:
Zyla 4.2 PLUS
Gallery:
Further Reading:
Application Note 1 | Application Note 2 | Case Study 1
Total Internal Reflection (TIRF) Microscopy
TIRFM makes use of an optical effect that can be adapted to observe fluorescent events occurring at the interface between two optical media of different refractive indices. TIRFM is limited to the area within a few hundred nanometres
of the glass/sample interface, where total internal reflection is occurring. TIRFM is an increasingly popular technique for visualizing, with high signal to background ratio, processes that occur in and around the membrane of living
cells. Both EMCCD cameras and sCMOS (Zyla 4.2 PLUS as well as the new Sona 4.2) can be utilised for TIRF Microscopy. The sensitivity of the EMCCD is relevant when the fluorophores of choice are emitting a small number of photons,
however when the photon emissions are sufficiently high, Zyla and Sona achieve excellent resolution with a large field of view combined with 95% QE and deep cooling of the new sCMOS camera.
Solutions for TIRF Microscopy:
iXon Life 897 | iXon Life 888 | Zyla 4.2 PLUS | Sona 4.2
Gallery:
Further Reading:
Application Note | Case Study
Ion Signalling Microscopy

The ability to monitor changes in intracellular ion concentrations over time is vital for our understanding of signalling and functional pathways in cellular systems. Ion channels that span the outer cell membrane open or close in
response to extracellular and intracellular signals, potentially altering how the cell behaves. These fluctuations can be visualized and quantified using ratiometric microscopy and special fluorescent dyes designed to bind specific
ions. iXon EMCCD models ensure maximum sensitivity under fast frame rates, low dye concentrations and reduced illumination. Speed, resolution and field of view can be pushed to the max with the sCMOS cameras. Zyla 5.5 with Global
Shutter exposure mode maintains temporal correlation across all areas of the sensor, e.g. when monitoring calcium sparks following electrical stimulation. Sona sCMOS with their 95% QE will be ideal for reduced dye concentrations
– and emitted signal levels in ratiometric experiments.
Solutions for Ion Signalling Microscopy:
iXon Life 897 | iXon Life 888 | Zyla 4.2 PLUS | Zyla 5.5 |
Neo 5.5 | Sona 4.2 | Sona 2.0
Gallery:
Further Reading:
Application Note | Case Study
Wide-Field Fluorescence Microscopy

The application of an array of fluorochromes has made it possible to identify cells and sub-microscopic cellular components with a high degree of specificity amid non-fluorescing material. In fact, the fluorescence microscope is capable
of revealing the presence of a single molecule. Through the use of multiple fluorophores, different probes can simultaneously identify several target molecules. Although the fluorescence microscope cannot provide spatial resolution
below the diffraction limit of specific specimen features, the detection of fluorescing molecules below such limits is readily achieved. The large field of view (2.0/4.2/5.5 MP) and the excellent resolution (choice of 6.5 or 11
µm pixel size) of sCMOS make it an ideal detector for wide-field fluorescence microscopy.
Solutions for Wide-Field Fluorescence Microscopy:
Neo 5.5 sCMOS | Zyla 5.5 | Zyla 4.2 PLUS | Sona 4.2 | Sona 2.0
Gallery:
Further Reading:
Application Note
Cell Motility

Cell movements involved in cell polarity, adhesion, membrane ruffling are just a handful of phenomena that are critical for complex processes including axonal guidance, tissue regeneration and morphogenesis. At the level of single
cell visualization, cell motility envelopes a broad area of study comprising the mechanisms behind unregulated cell growth and propagation during metastatic cancer changes. Cytoskeletal dynamics and membrane morphology of moving
cells need imaging with high resolution and sensitivity, such that the underlying mechanisms are preserved in the living cell for as long as possible, through minimization of phototoxic damage and photobleaching of the fluorophores.
While in extremely low light conditions where the raw sensitivity of EMCCD excels, Andor sCMOS family offers a selection of cameras well suited to the demands of large field-of-view, high-resolution, rapid frame rate imaging of
motile cells specimens.
Solutions for Cell Motility:
iXon Life 897 | iXon Life 888 | Neo 5.5 | Zyla 5.5
Gallery:
Further Reading:
Case Study 1
Spinning Disk Confocal Microscopy

Spinning disk confocal microscopy utilizes multiple pinholes to project a series of 1000+ parallel excitation light beams onto the specimen in a multiplexed pattern that is subsequently detected after fluorescence emission passes through
the same pinholes. This technique is highly useful for high speed imaging of living cells expressing fluorescent proteins. While photobleaching and phototoxicity are reduced, a tighter light budget requires very sensitive camera
systems for optimum signal detection. Thus EMCCD cameras with their EM gain functionality are the detectors of choice for this application and allow the user to preserve their living specimens and therefore image for longer. For
applications that do not require single photon sensitivity, Andor sCMOS cameras are the perfect match thanks to their combination of field of view, speed, sensitivity and pixel size. Andor Zyla or the new Sona sCMOS deliver up
to 100 fps and offer unparalleled resolution and image quality.
Solutions for Spinning Disk Confocal Microscopy:
iXon Life 897 | iXon Life 888 | Sona 4.2 | Sona 2.0
Gallery:
Further Reading:
App Note 1 | App Note 2 | Case Study 1 | Case Study 2 | Case Study 3
FRAP and FRET

Fluorescence Recovery After Photobleaching (FRAP) denotes an optical technique capable of quantifying 2D lateral diffusion of a molecularly thin film containing fluorescently labelled probes or to examine single cells. This technique
is very useful in biological studies of cell membrane diffusion and protein binding. Fluorescence Resonance Energy Transfer (FRET) is a microscopy technique used to measure the proximity of two fluorophores. Resonance energy transfer
occurs only over very short distances, typically within 10 nm, and involves the direct transfer of excited state energy from the donor fluorophore to an acceptor fluorophore as an alternative to fluorescence emissive decay from
the donor. Offering high resolution, high speed and high S/N, EMCCDs are the cameras of choice for FRAP and FRET imaging.
Solutions for FRAP and FRET:
iXon Life 897 | iXon Life 888 |
Gallery:
Further Reading:
Application Note 1 | Application Note 2 | Case Study
Live Cell Imaging

Live Cell Imaging now spans multiple modalities, including widefield (fluorescence, phase contrast, and differential interference contrast), laser scanning confocal, multiphoton, and spinning disk microscopy. It is used to provide
critical insight into the fundamental nature of cellular and tissue function through the study of cellular dynamics. The EM gain capability of iXon EMCCD is a necessary requirement to ensure the living cell remains living for the
duration of the experiment and special features such as 'Crop mode' acquisition provide fast frame rates for dynamic events. Alternatively the speed and field of view of sCMOS make it ideal for live cell imaging. Zyla 5.5 with
Global Shutter exposure mode provides a capability that is both 'photon-efficient' and easy to synchronize to, especially critical for 3D / 4D microscopy. The Sona sCMOS offers 95% QE and will be an ideal choice for more weakly
emitting samples that may be susceptible to photdamage from longer light exposures.
Solutions for Live Cell Imaging:
iXon Life 897 | iXon Life 888 | Neo 5.5 | Zyla 5.5 | Sona 4.2
Gallery:
Further Reading:
Case Study 1 | Case Study 2
Bioluminescence

Bioluminescence refers to the ability of living things to produce light and represents a profoundly useful area of biophotonic research. A well-known bioluminescence is that induced by the luciferase enzyme. For example, firefly luciferase
emits light in the presence of its substrate luciferin & adenosine triphosphate (ATP) and is widely used for measuring ATP concentrations. Low read noise, low dark current and a high QE are key parameters for a bioluminescence
detector. Deep cooled CCD cameras are suited to ultrasensitive imaging of bio/chemiluminescence. They offer ultra-low dark noise due to exceptional TE Cooling and are ideal if longer exposures are required for weaker bioluminescent
signals.
Solutions for Bioluminescence:
iKon-M 934 | iXon Life 888 | iXon Life 897
Further Reading:
Case Study 1